Product Uses
Measurement of calcium activated myosin ATPase activity when bound to thin filaments.
Identification/characterization of proteins or small molecules that affect the TT complex regulation and myosin ATPase activity
Identification/characterization of proteins or small molecules that affect myosin / F- actin interaction
Materials
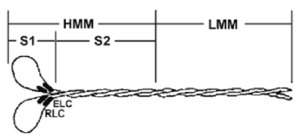
Cardiac myosin protein has been purified from bovine heart tissue(1, 2). The full length myosin protein was purified with its essential light chains (ELC) and regulatory light chains (RLC), see Figure 1and 2. Myosin was then digested with alpha-chymotrypsin to liberate the soluble subfragment-1 (S1) domain, which was isolated by centrifugation (3). The purified myosin S1 fragment has been determined to be biologically active in an F-actin activated ATPase assay (see biological activity assay). Bovine cardiac myosin S1 fragment protein is supplied as a white lyophilized powder.
Figure 1. Diagrammatic representation of the myosin protein and its subfragments