Product Uses Include
Measurement of F-actin activated myosin ATPase activity
Identification/characterization of proteins or small molecules that affect myosin II ATPase activity
Identification/characterization of proteins or small molecules that affect the myosin II - F-actin interaction
Control myosin ATPase activity in drug discovery screening projects
Material
Myosin II protein has been purified from rabbit skeletal muscle. The full length myosin II protein has been purified with its essential light chains (ELC) and regulatory light chains (RLC), see Figure 1 and 2. Myosin II has been determined to be biologically active in an F-actin activated ATPase assay. Rabbit skeletal muscle myosin II is not recommended for use in motility assays. Rabbit myosin II protein is supplied as a white lyophilized powder. When reconstituted in nanopure water, the protein will be in the following buffer: 25 mM PIPES-NaOH pH 7.0, 1.25 M KCl, 2.5% sucrose and 0.5% dextran. The lyophilized protein is stable at 4°C desiccated (<10% humidity) for 1 year.
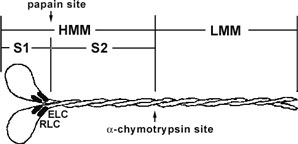
Figure 1. Diagrammatic representation of the myosin II protein and its subfragments. Myosin II or conventional myosin is a hexameric protein consisting of two heavy chains and two light chains. Myosin II can be proteolytically cleaved into heavy meromyosin (HMM, Cat.# MH01) and light meromyosin (LMM) by α-chymotrypsin. Heavy meromyosin consists of the myosin head subfragment-1 domain (S1), its associated light chains (essential light chains and regulatory light chains), and the coiled-coil subfragment -2 domain. Light meromyosin consists of coiledcoil protein structure. The myosin S1-subfragment is produced by papain digestion of HMM.